 Welcome to Chemistry 212 — Survey of Organic and Biochemistry, section DE taught by Prof. J. Walker, Fall 2010, Truman College. If you are enrolled in this section please check this website frequently for announcements and new information. Section DE meets Monday and Wednesday from 12:30 p.m. to 3:15 p.m. in room 3831 beginning Monday August 23rd. Dr. Abrams is also an instructor for Chemistry 212. I recommend that all students visit Dr. Abrams' website. He has an extensive collection of practice quizzes and exams including a readiness/review quiz..
Welcome to Chemistry 212 — Survey of Organic and Biochemistry, section DE taught by Prof. J. Walker, Fall 2010, Truman College. If you are enrolled in this section please check this website frequently for announcements and new information. Section DE meets Monday and Wednesday from 12:30 p.m. to 3:15 p.m. in room 3831 beginning Monday August 23rd. Dr. Abrams is also an instructor for Chemistry 212. I recommend that all students visit Dr. Abrams' website. He has an extensive collection of practice quizzes and exams including a readiness/review quiz..
Course Catalog Description
CHEMISTRY 073 0212 - Survey of Organic and Biochemistry
Survey of organic chemistry including nomenclature and reactions of major functional groups essential to biochemistry and an introduction to the structure and function of biomolecules, and the metabolism of proteins, lipids, and carbohydrates. Writing assignments, as appropriate to the discipline, are part of the course.
Prerequisites: Chemistry 201 or consent of department chairperson. 3 lecture hours and 3 lab hours per week based on sixteen weeks. Credit Hours: 4.
Instructor
 Prof Joy Walker
Prof Joy Walker
Learning About Volcanos at Crater Lake
I'm a professor emeritus for the City Colleges of Chicago. I was a tenured professor for 30+ years at Truman College. I love to learn and share my enthusiasm for learning. I have many interests. I enjoy learning about science, gardening, cooking and baking, linguistics and languages, drawing, painting and crafting, and literature. I believe we learn best when we are curious, excited, interested and engaged in our own learning processes.
Textbooks
Introduction to Organic and Biochemistry 7th ed.
 by Bettelheim, Brown, Campbell and Farrell
by Bettelheim, Brown, Campbell and Farrell
Thomson ©2010
ISBN: 0-495-39116-6
Companion site for textbook: Free flashcards, quizzes, active figures etc.
pdf of Chapter One is free
note: It is possible to use the 6th edition of this book.
Laboratory Experiments for General, Organic, and Biochemistry, 7th Edition
 by Bettelheim and Landesberg
by Bettelheim and Landesberg
Thomson ©2010
ISBN: 0-495-39196-4
We only use a few of the many experiments in this book. It is cheaper to purchase individual experiments online at Cengage Brain. You will need the following chapters, in order of use in class:
- 21: Structure in Organic Compounds: Use of Molecular Models I
- 24: Classification and Identification of Hydrocarbons
- 25: Classification and Identification of Alcohols and Phenols
- 22: Stereochemistry: Use of Molecular Models II
- 18: pH and Buffer Solutions
- 28: Properties of Amines and Amides
- 26: Classification and Identification of Aldehydes and Ketones
- 34: Preparation and Properties of Soap
- 32: Carbohydrates
- 40: Isolation and Identification of Casein
- 31: Isolation of Caffeine from Tea Leaves
Cengage Learning Sign In to access online textbook resources
Model Kit
Although a model kit is not required, it is strongly recommended that you obtain one, and you will need it in the first week of class. Please refer to recommendations by Dr. Abrams when chosing a suitable model kit. This class involves lots of drawing chemical structures. Colorful pencils/pens are useful while taking notes.
Favorite Molecule
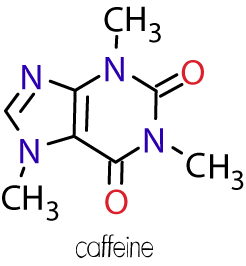
Caffeine has the molecular formula C8H10N4O2 and acts as a stimulant in humans. It is found in many beverages such as coffee, tea, cola and energy drinks. The name comes from the Italian term for coffee, caffè. Read what Wikipedia has to say about caffeine.

Recommended Links
- Textbook Website
- Understanding Chemistry
- Doc Brown's Chemistry Clinic
- ACD Labs ChemSketch Freeware
- Virtual Textbook of Organic Chemistry
- The Chemogenesis Web Book
- C60, the Celestial Sphere that Fell to Earth
Who's Who
- Friedrich August Kekulé von Stradonitz— He was the principal founder of the theory of chemical structure and most famous for his work on the structure of benzene.
- Friedrich Wöhler — He accidentally synthesized urea in 1828 and showed it was possible to artificially prepare organic compounds.